|
Final Diagnosis:
Heterobilharzia americana
Clinical
History: A 5-year-old intact female
Shih-Tzu presented for evaluation of vomiting, diarrhea, and decreased appetite.
She was in heat approximately one month previous to onset of clinical signs.
Radiographs of the abdomen revealed mildly distended bowel loops. A
presumptive clinical diagnosis of pyometra was made and an exploratory
laparotomy was performed.
Surgical Findings: Laparotomy revealed a mildly turbid fluid within the
peritoneum. Multiple firm nodules (approximately 0.5 cm in diameter) were
present in the right lobe of the pancreas, small intestinal serosa and enlarged
mesenteric lymph nodes. The uterus was mildly distended by fluid. Biopsy
specimens from the pancreas, lymph node, and jejunum were submitted for
histopathology. An ovariohysterectomy was performed after the biopsies and
uterus and ovaries were also submitted for histologic evaluation.
Histologic Findings: The lymph node contained numerous round to oval,
approximately 80 µm wide ova with a yellow to tan, thick hyalinized wall
distributed primarily in the paracortex. Many of the ova contained developing
miracidium, although a few were empty. A marked inflammatory reaction
surrounded individual ova and consisted of many epithelioid macrophages with
fibrosis, eosinophils, neutrophils, lymphocytes, plasma cells, and few
multinucleated giant cells.
The small intestine
(jejunum) contained ova and granulomatous inflammation distributed transmurally
and near venules, likely arborizing from the mesenteric veins. Similar ova and
granulomatous inflammation was observed in the pancreas.
Uterus and ovaries were
histologically unremarkable.
Ancillary testing: Fecal sedimentation in saline revealed
miracidia-laden eggs.
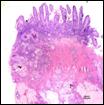 Small
intestine with transmural parasitic ova containing miracidium and surrounded by
granulomatous inflammation. Eggs are consistent with Heterobilharzia
americana. Small
intestine with transmural parasitic ova containing miracidium and surrounded by
granulomatous inflammation. Eggs are consistent with Heterobilharzia
americana.

Mesenteric lymph node with
numerous parasitic ova and surrounded by granulomatous inflammation. Eggs are
consistent with Heterobilharzia americana.
 Miracidia-laden trematode
egg consistent with Heterobilharzia americana from fecal
sedimentation. Note the miracidium within a thick refractile capsule and no
operculum. Miracidia-laden trematode
egg consistent with Heterobilharzia americana from fecal
sedimentation. Note the miracidium within a thick refractile capsule and no
operculum.
Discussion: Heterobilharzia americana, classified as a schistosome, is a blood fluke of wild
and domestic carnivores and is widespread in southern Atlantic and gulf coast
states (Florida, Louisiana, Mississippi, Texas, Georgia, North Carolina, South
Carolina) as well as east central states and southeast Kansas. The life cycle
of H. americana is indirect, involving an aquatic snail (Lymnaea
cubensis and Pseudocuccinea columella) as the intermediate host.
Cercariae that are released from the snail intermediate host infect dogs and
wildlife through direct skin penetration. Clinically significant H. americana infection includes massive inflammation leading to intestinal disorders,
dehydration, pancreatic insufficiency, and systemic dissemination. Antemortem
diagnosis requires fecal sedimentation in 0.9% NaCl to identify characteristic
trematode eggs. Schistosome ova are large (70-90µm), non-operculated, and
contain miracidium. Unfortunately, schistosome larvae were not identified in
multiple tissue sections. Diagnosis was based on fecal sedimentation by the
Clinical Parasitology Laboratory at Purdue University as well as
histopathologic evaluation of ova. The dog was presumably infected while
vacationing in Florida in July and subsequently became ill in August of the
same year. Treatment with fenbendazole for 14 days was initiated and repeated
after three weeks. One month following administration of high doses of
fenbendazole, no ova were detected on sedimentation, feces were formed, and
appetite and activity were normal.
-by Dr. Abigail Durkes,
ADDL Graduate Student
References:
-
Flowers J, Hammerberg B et al:
2002. JAVMA 220(2):193-96.
-
Goff W and Ronald J: 1982.
American Journal of Veterinary Research 42:1775-76.
-
Malek E, Ash L et al: 1961.
Journal of Parasitology 47:619-23.
-
McKown R, Veatch J et al:
1991. Journal of Wildlife Diseases 27(1): 156
|